The most common adverse reactions reported in clinical trials (>0.5% of subjects) were Factor VIII inhibition in previously untreated patients (PUPs), dizziness, and hypersensitivity.
Paresthesia
Rash
Erythema
Pruritus
Pyrexia
Injection-site pain
Chills
Feeling hot
Dizziness
Hypersensitivity
One patient withdrew due to an adverse reaction (hypersensitivity).
*Adverse reactions reported in adult/adolescent and pediatric studies in previously treated patients
The one-stage clotting assay result underestimates the Factor VIII activity level compared to the chromogenic assay result by approximately one-half.
If the one-stage clotting assay is used, multiply the result by a conversion factor of 2 to determine the patient’s Factor VIII activity level.
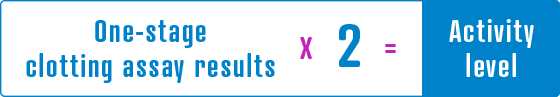
Data from three AFSTYLA studies: Safety of AFSTYLA was assessed in patients enrolled in the phase I/III, open-label, multicenter, crossover study, the phase III, open-label study, and the ongoing phase III, open-label, multicenter extension study. Adverse reactions were reported for 14 of 258 (5.4%) patients in all studies. In the clinical studies, there have been a total of 28,418 exposure days, with at least 28,492 injections of AFSTYLA administered.