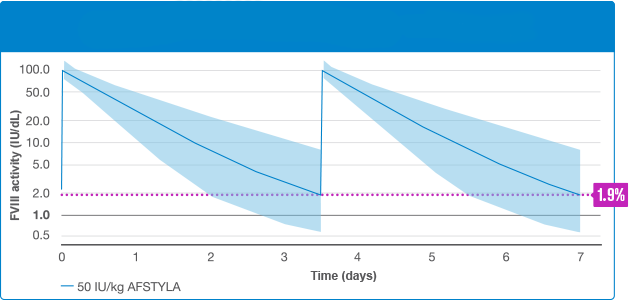
With twice-weekly dosing†1:
80% of patients could maintain >1% FVIII activity
When dosed three times weekly†1:
96% of patients could maintain >1% FVIII activity
Data derived from simulated steady-state analysis from a PK model developed based on the levels of FVIII activity in previously treated adolescent and adult patients who had FVIII activity <1 IU/dL and no FVIII inhibitors at screening. Patients had not received FVIII therapy for at least 4 days prior to being given AFSTYLA at 50 IU/kg. Blood samples for PK assessments were collected before infusion (predose) and up to 96 hours after infusion. A visual predictive check was performed to evaluate the performance of the final population PK model.
"One less infusion means a world of difference to a parent."‡
–Hector, whose son Jonathan switched to AFSTYLA
‡Testimonial is the speaker’s personal experience. Individual experiences will vary.
The recommended starting regimen for routine prophylaxis is:
Adjust regimen based on patient response
FDA approved for dosing 2 to 3 times a week
The median doses used in AFSTYLA clinical trials were
§Annualized spontaneous bleeding rate in clinical trials (IQR=0–2.4 for patients ≥12 years; 0–2.2 for patients <12 years).

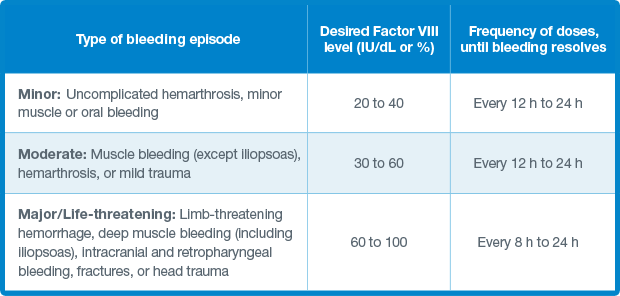
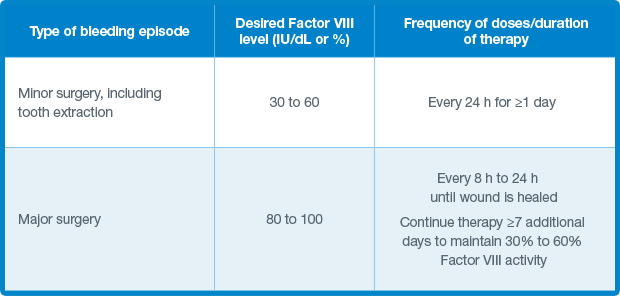
Reference: 1. Zhang Y, Roberts J, Tortorici M, et al. Population pharmacokinetics of recombinant coagulation factor VI 11-SingleChain in patients with severe hemophilia A. J Thromb Haemost. 2017;15(6):1106-1114.
The dose to achieve a desired in vivo peak increase in Factor VIII level may be calculated using the following formula:

The amount of AFSTYLA to be administered and the frequency of administration should always be oriented to the clinical effectiveness in the individual case.