
“As a paramedic...it's an active job. Being able to work and be protected by my factor product is huge." ‡
–Cody switched to AFSTYLA
‡Testimonial is the speaker's personal experience. Individual experiences will vary.
AFSTYLA was tested in the largest FVIII pivotal clinical trial program to date, with 258 previously treated patients with severe hemophilia A (226 received prophylaxis).

Phase I/III, open-label, multicenter, crossover study in patients ≥12 years, and a phase III, open-label, multicenter study in patients <12 years.
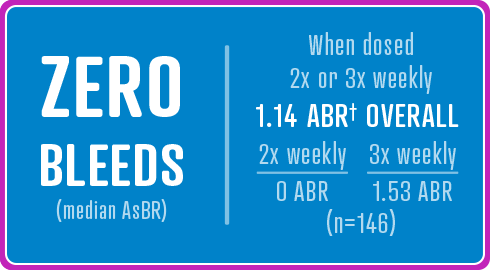
175 previously treated male patients with severe hemophilia A (<1% endogenous Factor VIII activity) ages 12 to 65 years, including 14 adolescent patients 12 to 18 years. 174 patients received at least one dose of AFSTYLA and 173 (99%) were evaluable for efficacy. A total of 161 patients (92.5%) completed the study.
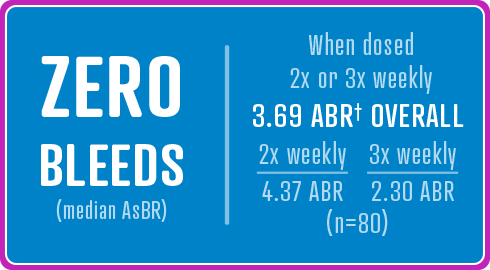
84 previously treated male children (35 patients 0 to <6 years and 49 patients ≥6 to <12 years) with severe hemophilia A (<1% endogenous Factor VIII activity). Of the 84 enrolled patients, all received at least one dose of AFSTYLA and 83 (99%) were evaluable for efficacy.