Like naturally occurring Factor VIII, AFSTYLA binds to von Willebrand factor (VWF), which helps protect it from degradation in circulation.
In the AFSTYLA Phase 1 PK study:
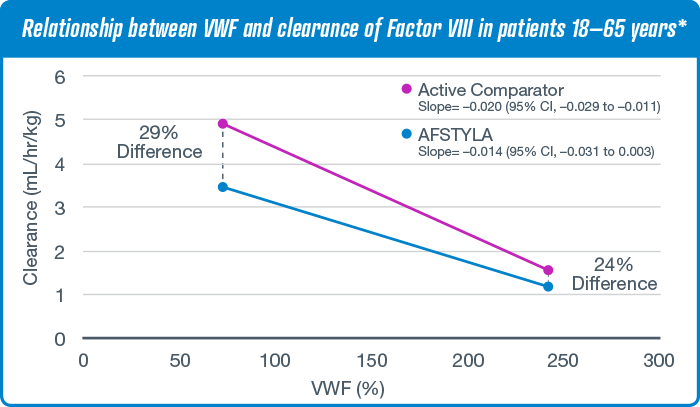
Clearance is mathematically related to other pharmacokinetic measures, such as half-life and area under the curve—important parameters that are considered when determining optimal dosing regimens.
*Study details
Samples from 27 adult patients were analyzed for predose VWF and clearance of Factor VIII after a single 50 IU/kg infusion of AFSTYLA and active comparator (with washout period). Regression lines estimated from a post hoc analysis are shown. 95% CI=95% confidence interval.2,3
References: 1. Zollner S, Raquet E, Claar P, et al. Non-clinical pharmacokinetics and pharmacodynamics of
rVlll-SingleChain, a novel recombinant single-chain factor VIII. Thromb Res. 2014;134(1):125-131. 2. Data on
file. Available from CSL Behring as DOF AFS-002. 3. Klamroth R, Simpson M, von Depka-Prondzinski M, et al.
Comparative pharmacokinetics of rVlll-SingleChain and octocog alfa (Advate®) in patients with severe
haemophilia A. Haemophilia. 2016;22(5):730-738.