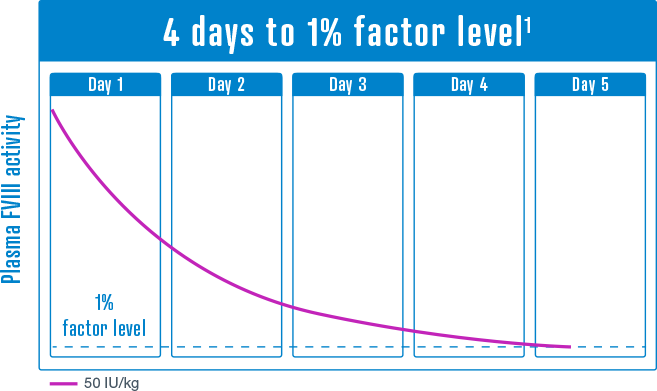
 50 IU/kg
50 IU/kg
Median time to trough levels of 1% FVIII activity has been estimated between 4 and 5 days, depending on dose and extrapolation method.
Reference: 1. Data on file available from CSL Behring as DOF AFS-001.

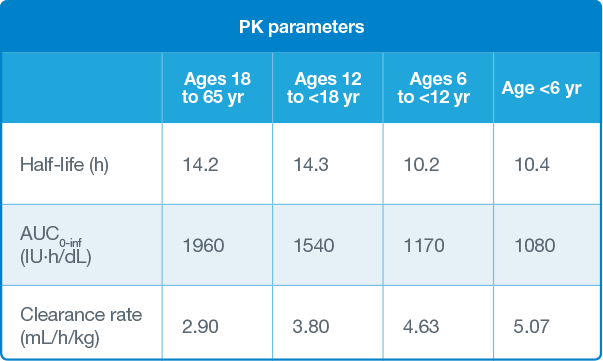
AUC and clearance rate parameters illustrate the enhanced bioavailability of AFSTYLA in circulation.
Phase I/III, open-label, multicenter, crossover study in patients ≥12 years, and a phase III open label, multicenter study in patients <12 years.
The pharmacokinetics (PK) of AFSTYLA was evaluated in 91 (81 adults and 10 adolescents) previously treated patients (PTPs) with severe hemophilia A (<1% endogenous Factor VIII activity) following an intravenous injection of a single dose of 50 IU/kg.
The PK of AFSTYLA was evaluated in 39 previously treated children (<12 years) with severe hemophilia A (<1% endogenous Factor VIII activity) following a 50 IU/kg intravenous injection of AFSTYLA.